Drugs approved for marketing by the FDA in January 2023
According to the public information from the FDA website [1], the drugs which were approved for marketing by the FDA's Center for Drug Evaluation and Research (CDER) in January 2023 were interpreted. 4 drugs were approved for marketing by the FDA in January 2023: 3 small molecules and 1 monoclonal antibody. Most of the following text content is referenced from the official FDA drug instructions, and some of the data are generally referenced from Wikipedia and official announcements of drug companies unless specifically cited.
1/6/2023:
Leqembi (lecanemab-irmb)
Basic information: Leqembi is a monoclonal antibody targeting amyloid beta jointly developed by Eisai and Biogen for the treatment of Alzheimer's disease. Alzheimer's disease is a progressive, irreversible brain disorder that affects over 6.5 million Americans. The precise cause of Alzheimer's disease is unknown, but it is characterized by amyloid plaques and neurogenic fiber or tau protein tangles, which result in the loss of neurons and their connections. These changes have an impact on a person's memory and thinking ability. leqembi slows the progression of neurodegenerative disease by removing harmful amyloid plaques from the brain. in June 2021, eisai and Biogen's Aduhelm monoclonal antibody became the FDA's first for Alzheimer's disease since 2003, but it has been controversial since its launch, arguing that current clinical evidence does does not show that the drug slows cognitive decline in patients.
Clinical Performance: Previously, researchers assessed the efficacy of Leqembi in a double-blind, placebo-controlled study of 856 Alzheimer's disease patients. Treatment was started in patients with mild cognitive impairment or mild dementia who had amyloid pathology confirmed. When compared to the placebo group, patients receiving approved doses of Leqembi (10 mg/kg every two weeks) had statistically significant reductions in brain amyloid plaques from baseline to week 79. Infusion-related reactions, headache, and ARIA (amyloid-related imaging abnormalities, including brain swelling and risk of brain hemorrhage) were the most common side effects of Leqembi [2].
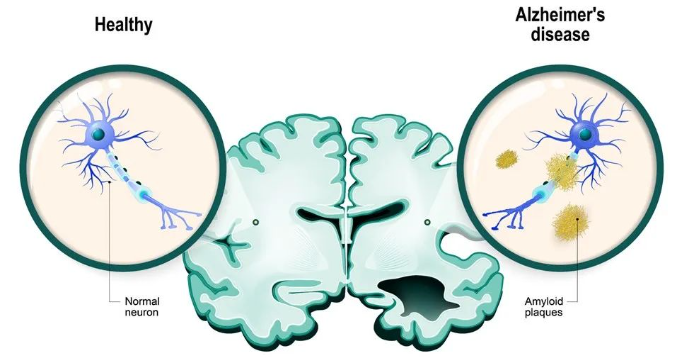
Figure 1. Comparison of neurons in healthy individuals and Alzheimer's disease patients. Image source:
https://www.rdworldonline.com/alzheimer-plaque-affects-different-brain-cells-differently/1/27/2023: Jayprica (Pirtobrutinib)Basic Info: Pirtobrutinib is a non-covalent BTK kinase inhibitor developed by Lilly (the drug was originally developed by Redx Pharma and later acquired by Loxo Oncology for $40 million, with Lilly acquiring Loxo in 2019 for approximately $8 billion) for the treatment of adult patients receiving at least second-line systemic therapy (including chemotherapy). BTK is a B-cell antigen receptor (BCR) and cytokine receptor signaling protein. BTK signaling in B cells activates pathways required for B cell proliferation, trafficking, chemotaxis, and adhesion. Pirtobrutinib binds to both wild-type and C481 BTK, reducing kinase activity. Pirtobrutinib decreased BTK-mediated B cell CD69 expression and lowered malignant B cell growth in non-clinical trials.The FDA granted clearance based on data from a sample of patients in the single-arm BRUIN study, with effectiveness assessments based on 120 MCL patients. 93% of these patients had undergone two or more therapies, and all had previously received one or more covalent BTK inhibitors. The overall remission rate (ORR) of treated MCL patients was 50%, with an 8.3-month median duration of remission (DOR) [4].
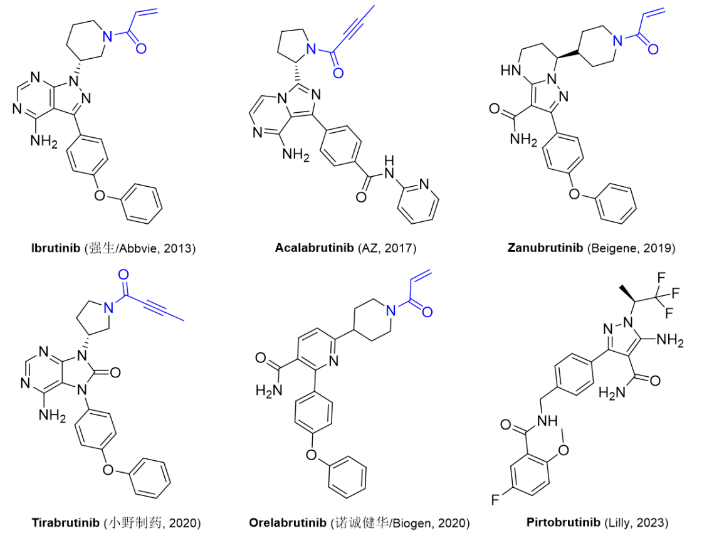
Figure 2. currently approved and marketed BTK inhibitors.
Ibrutinib CAS: 936563-96-1Acalabrutinib
CAS: 1420477-60-6Zanubrutinib CAS: 1691249-45-2
Tirabrutinib
CAS: 1351636-18-4
Orelabrutinib CAS: 1655504-04-3
| Pirtobrutinib CAS: 2101700-15-4 |
1/20/2023:
Brenzavvy (bexagliflozin)
Basin Info: TheracosBio developed bexagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, to improve glycemic control in adults with type 2 diabetes. Several SGLT2 inhibitors have been marketed, with Empagliflozin and Dapagliflozin holding the highest market shares, with sales of $5.829 billion and $3.274 billion, respectively, in 2021. SGLT2 is primarily expressed in the kidney and is responsible for the reabsorption of filtered glucose from the renal tubular lumen Bexagliflozin is also the first SGLT2 inhibitor approved by the FDA for use in cats.
Clinical Performance: FDA approval was based on a Phase 3 clinical study in more than 5,000 patients with type 2 diabetes. Whether used as monotherapy, in combination with metformin, or as a supplement to standard of care therapy consisting of multiple regimens (including metformin, sulfonylureas, insulin, and DPP4 inhibitors), Bexagliflozin significantly reduced hemoglobin A1c and fasting glucose after 24 weeks. Although Bexagliflozin is not approved for weight or blood pressure reduction, modest reductions in body weight and systolic blood pressure have been observed in the clinic [3].
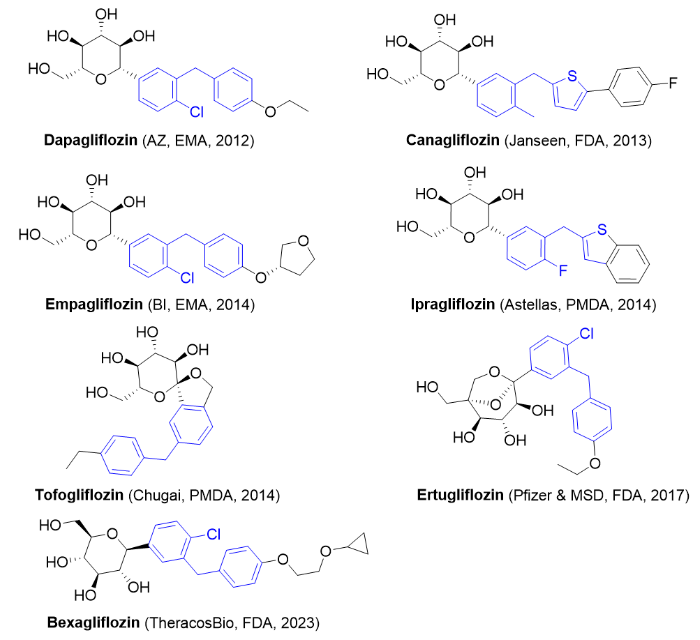
Figure 3. Some of the SGLT2 inhibitors currently approved for marketing.
Dapagliflozin CAS: 461432-26-8Canagliflozin CAS: 842133-18-0Empagliflozin
CAS: 864070-44-0
Ipragliflozin CAS: 761423-87-4
Tofogliflozin CAS: 903565-83-3
Ertugliflozin CAS: 1210344-57-2
Bexagliflozin CAS: 1118567-05-7 |
1/27/2023: Orserdu (elacestrant )
Basic info: Stemline Therapeutics (a subsidiary of the Italian pharmaceutical company Menarini Group) developed elacestrant, an oral estrogen receptor antagonist (Selective Estrogen Receptor Degrader (SERD)), for the treatment of advanced or metastatic breast cancer with ER+/HER2-, ESR1 mutations after at least one endocrine therapy. Postmenopausal women or adult males with advanced or metastatic breast cancer that has progressed due to ER+/HER2-, ESR1 mutations. Elacestrant inhibits 17-estradiol-mediated cell growth in ER+/HER2- breast cancer cells at dosages that cause ER protein degradation via the proteasome pathway. Elacestrant has anticancer effect in vitro and in vivo, including in ER+/HER2- breast cancer models resistant to fulvestrant, CDK4/6 inhibitor models, and models with estrogen receptor 1 gene alterations (ESR1). Previously, only fulvestrant was available for SERD in 2002 and required intramuscular administration.
Clinical performance: The FDA granted clearance based on effectiveness in the EMERALD Phase III study, which included 478 postmenopausal women and men with advanced or metastatic ER+/HER2- breast cancer, 228 of whom had an ESR1 mutation. Patients were randomly randomized (1:1) to either once-daily oral Elacestrant (n=239) or investigator-selected endocrine treatment, which comprised either fulvestrant (n=166) or an aromatase inhibitor (n=73), with progression-free survival serving as the major effectiveness endpoint (PFS). The median PFS was 3.8 months in the Elacestrant group and 1.9 months in the fulvestrant or aromatase inhibitor group among 228 (48%) patients with ESR1 mutations [5].
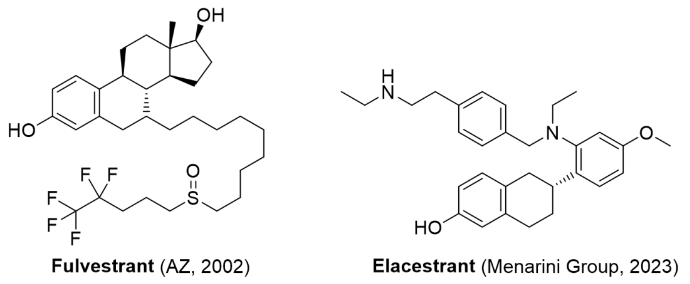
Figure 4: Selective estrogen receptor antagonists that are currently approved for the market.
Fulvestrant CAS: 129453-61-8Elacestrant
CAS: 722533-56-4


