NASH treatment drug hits high note: Resmetirom Phase III progress excellent
A total of four MEK inhibitors, including semitinib (AstraZeneca/Mercedon), binitinib (Pfizer/Pierre Fabre/Ono Pharmaceutical), cobimetinib (Roche/Exelixis), and trametinib (Japan Tobacco/Novartis), have been authorized for marketing globally as of this point. Trametinib began selling in China in December 2019. Several domestic pharmaceutical firms have developed MEK inhibitors, including KZP, Hengrui Pharmaceuticals, Fultronics, and Zhengda Tianqing. Trametinib, developed by KZP, was announced to be listed in China on January 29 of this year, and SHR7390 and FCN-159, developed by Hengrui Pharmaceuticals and Fultronics, respectively, are in the Phase II clinical stage.
A total of four MEK inhibitors, including semitinib (AstraZeneca/Mercedon), binitinib (Pfizer/Pierre Fabre/Ono Pharmaceutical), cobimetinib (Roche/Exelixis), and trametinib (Japan Tobacco/Novartis), have been authorized for marketing globally as of this point. Trametinib began selling in China in December 2019. Several domestic pharmaceutical firms have developed MEK inhibitors, including KZP, Hengrui Pharmaceuticals, Fultronics, and Zhengda Tianqing. Trametinib, developed by KZP, was announced to be listed in China on January 29 of this year, and SHR7390 and FCN-159, developed by Hengrui Pharmaceuticals and Fultronics, respectively, are in the Phase II clinical stage.
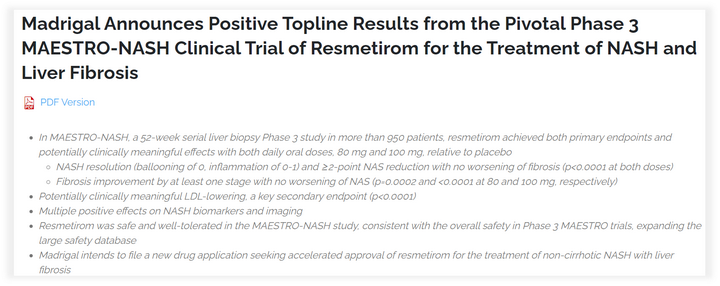
Up until recently, news of a development in NASH clinical progress broke the impasse in NASH medication development and gave many pharmaceutical developers newfound hope. Resmetirom (MGL-3196), a THR-agonist, met both its primary and important secondary goals in Phase III clinical trials, according to a Madrigal announcement. Resmetirom had already demonstrated in Phase II great therapeutic potential, including decreased liver lipids, enhanced metabolic and inflammatory responses, and clean safety metrics, and now it is finally moving forward.
Resmetirom significantly outperformed the placebo group for both dose NAS (NAFLD activity score) decreases of more than 2 points, significantly outperformed the placebo group for at least one stage of fibrosis improvement, and significantly outperformed the placebo group for LDL-C decreases in important secondary endpoints in this Phase III clinical study with publicly available data.

The results are positive and are said to have inspired other NASH medication development firms. A proper preclinical animal model is crucial for the discovery of new drugs. In order to reduce the discrepancy between preclinical and clinical results of drugs and increase the effectiveness of drug development, selecting an appropriate NASH model can not only significantly reduce modeling time but can also simulate the characteristics of metabolic disorders and liver pathology histology of human patients.
Mouse model for replicating human NASH disease
B6-Alms1-del(T017633)
Alström syndrome is linked to the ALMS1 gene, which is also linked to metabolic syndromes such childhood obesity, developmental delay, hyperlipidemia, cardiomyopathy, and type II diabetes mellitus. The mice have a mixed phenotype of alcoholic fatty liver disease, diabetes, and obesity. Feeding on a Western diet (WD) speeds up the occurrence of metabolic problems and liver damage, and it may result in mild liver fibrosis. Because it may significantly shorten the modeling time compared to the conventional NASH model, B6-Alms1-del NASH is a good model with both metabolic problems and liver pathology and can be utilized for preclinical efficacy assessment of anti-NASH medications.

B6-Alms1-del mice fed on normal chow (CD) developed a fatty liver phenotype at 21 weeks of age, but the score was only 4.2 and did not reach the level of NASH. B6-Alms1-del mice fed on Western chow (WD) phenotyped significant steatosis, inflammation, ballooning lesions, and moderate fibrosis after 6 weeks. the NAS score was 6.0 and the fibrosis score was 1.6.
Pharmacodynamic testing of MGL-3196 in the B6-Alms1-del NASH model

MGL-3196 administration was able to significantly reduce lipid levels in WD-induced B6-Alms1-del NASH mouse model, and showed a dose-dependent effect, with more pronounced lipid-lowering effect in the high-dose group. (G1: B6J+Vehicle; G2: B6-Alms1-del NASH+Vehicle; G3: B6-Alms1-del NASH+MGL-3196 1mpk p.o.qd; G4: B6-Alms1-del NASH+MGL-3196 3mpk p.o.qd; G5: B6- Alms1-del NASH+MGL-


MGL-3196 administration alleviated the WD-induced steatosis and fibrosis phenotypes in the B6-Alms1-del NASH mouse model, and NAS scores and fibrosis scores were reduced to some extent and showed a dose-dependent effect.
Wild Mouse 750 (strain number: D000750)
Chromosome 1 from a wild mouse from the Yangpu region was inserted into the background of C57BL/6J mice, which have more genetic variation than inbred mice, to create the chromosomal replacement strain known as Wild Mouse 750. After 8 weeks of age, wild mice number 750 fed a regular diet showed a spontaneous obesity phenotype; this phenotype grew worse with each passing week, and a significant fatty liver phenotype was seen at 26 weeks of age. After 20 weeks of Western diet (WD) feeding, wild rats 750 showed characteristic NASH signs, including a steatosis and inflammation phenotype, as well as a moderate degree of fibrosis. This further hastened the appearance of NASH illness. Clinical NAS scoring standards state that a score of less than five is typically indicative of NASH, and wild mouse 750 scored more than 5 after 20 weeks of WD induction and therefore could be used as a NASH study. In addition this mouse has a fibrosis score of approximately 2, which can be used as an evaluation of antifibrotic drugs.
Wild mouse replacement lines, which have greater genetic variety and disease susceptibility than current inbred lines, are better at simulating the population because they carry mutations brought on by the environment and the natural world. The genetic diversity of wild mouse replacement lines can be employed in preclinical animal trials to better represent the efficacy and harmful effects of medications on various genetically described groups, and hence direct clinical experiments.

The 750 mice showed spontaneous obesity after 8 weeks of age, with significantly higher body weight than the B6 mice, and significantly more adipose tissue in all parts of the 750 mice than the B6 mice. Mesenteric (m), subcutaneous (s), epididymal (e), white adipose tissue (WAT), brown adipose tissue (BAT).
MGL-3196 efficacy test in wild rats 750


Reference from https://zhuanlan.zhihu.com/p/594389707








