3) 1 enzyme: α-mannosidase (for α-mannosidosis storage).
Informational Notes: A sodium-glucose co-transporter 2 (SGLT2) inhibitor called bexagliflozin was created by TheracosBio to help adults with type 2 diabetes better control their blood sugar levels. With sales of $5.829 billion and $3.274 billion, respectively, in 2021, Empagliflozin and Dapagliflozin have the greatest market shares among the SGLT2 inhibitors that have been put on the market. The SGLT2 enzyme, which is mostly expressed in the kidney, is in charge of reabsorbing filtered glucose from the renal tubular lumen. Bexagliflozin is also the first SGLT2 inhibitor for cats that the FDA has approved.
The natural substance bexagliflozin, which is often present in apple root bark, inhibits both SGLT1 and SGLT2, which are largely expressed in the intestine. Several SGLT2 selective inhibitors currently on the market are optimized based on the bexagliflozin structure.
Basic information: Lilly developed the non-covalent BTK kinase inhibitor pirtobrutinib for the treatment of adult patients who are receiving at least second-line systemic therapy. Pirtobrutinib binds to BTK wild-type and C481 mutants, thereby inhibiting BTK kinase activity. The drug was initially developed by Redx Pharma and later purchased by Loxo Oncology for $40 million.
Pirtobrutinib, a non-covalent inhibitor that targets mutations in BTK C481S (C481 is a covalent binding site for first-generation covalent inhibitors), is a proposal for a molecular design. There were no articles on Pirtobrutinib's drug discovery.
Basic informations: For the treatment of advanced or metastatic breast cancer with ER+/HER2-, ESR1 mutations following at least one endocrine therapy, Stemline Therapeutics, a division of the Italian pharmaceutical company Menarini Group, developed the oral estrogen receptor antagonist elacestrant (Selective Estrogen Receptor Degrader (SERD)). Elacestrant has shown antitumor activity in vitro and in vivo in postmenopausal women and adult men with advanced or metastatic breast cancer that has progressed after at least one endocrine therapy, including ER+/HER2- breast cancer models resistant to fulvestrant, CDK4/6 inhibitors, and models with mutations in the estrogen receptor 1 gene (ESR1). Prior to this, SERD could only use fulvestrant, which needed to be administered intramuscularly and had peak sales of $1 billion in 2002.
Ideas for molecular design There were no publications about drug discovery for elacestrant. Oral SERD development is by no means a simple process. The most anticipated contender in the clinical phase is AZ's camizestrant (AZD9833), which failed to satisfy the primary endpoint of PFS like Sanofi and Roche's prospects did.

2/1/2023: Jesduvroq (daprodustat)
Informational details: An oral HIF-PH (hypoxia-inducible factor-prolyl hydroxylase) inhibitor called daprodustat was created by GSK to treat anemia brought on by chronic kidney disease (CKD) in individuals who had received at least four months of dialysis. Similar to the physiological consequences that take place in humans at high altitudes, inhibition of HIF-PH stabilizes hypoxia-inducible factor, which in turn causes the transcription of erythropoietin and other genes implicated in the treatment of anemia.CKD, which affects 700 million people globally, is a major global health burden, with anemia thought to afflict 1 in 7 patients.In the US, CKD affects over 39 million people, of whom about 6 million also have anemia. In the US, there are about 810,000 persons who have end-stage renal disease (ESRD). Of these, 558,000 patients are on dialysis.
Prolyl hydroxylase (PH), an iron-containing dioxygenase, requires the substrate 2-oxoglutarate (2-OG) as well as iron as a cofactor. As a prototype, the 2-OG analog NOG was given a pyrimidinetrione backbone in order to produce daprodustat (GSK-1278863).

2/17/2023: Filspari (sparsentan)
Basic Information: Sparsentan is a once-daily oral drug developed by Travere Therapeutics that selectively targets endothelin-1 and angiotensin II (two key pathways in the progression of IgAN disease). It is the first non-immunosuppressive therapy for the treatment of IgAN disease, a rare progressive kidney disease (IgAN is the most common type of primary glomerulonephritis worldwide, affecting up to 150. Notably, Travere, whose notorious founder was Martin Shkreli, changed its name to its present name to distance itself from the relationship with its founder.
Sparsentan was initially created by BMS and passed through numerous hands before it was acquired by Travere in 2012. The AT1 receptor antagonist Irbesartan, which went on sale in 1997, and the ETA receptor antagonist BMS-193884, which was discontinued in phase 2, were combined to create sparsentan, which was then produced by optimizing the potency and oral bioavailability of the side chain and isoxazole sulfonamide fraction by SAR. The article "Sparsentan: From hypertension to kidney disease, how small biotechs are plucking gold from the sand of big BMS" has further information on sparsentan.

2/28/2023: Skyclarys (omaveloxolone)
Informational details: Reata Pharmaceuticals created the first-in-class oral drug omeveloxolone for the once-daily treatment of Friedreich's ataxia, a condition affecting people 16 years of age and older. Mutations in the gene that codes for the mitochondrial protein frataxin are the cause of Friedreich's ataxia. Loss of functioning frataxin causes mitochondrial malfunction, impairs iron-sulfur cluster production, and raises oxidative stress vulnerability. The condition results in progressive damage to the brain, spinal cord, and peripheral nerves, impairing balance, making it difficult to walk, altering speech and swallowing, and shortening lifespan.Omaveloxolone's therapeutic mechanism is uncertain, although it is generally accepted that this pentacyclic triterpene functions as an NRF2 activator by inhibiting KEAP1 and activating the antioxidant transcription factor NRF2. In the United States, an estimated 5,000 people suffer from Friedreich's ataxia.
Omaveloxolone is a pentacyclic triterpenoid molecule (cyanoenone triterpenoids), a byproduct of oleanolic acid, a natural substance that has biological properties like antioxidant, anti-inflammatory, and anti-apoptotic. The main goal of modification is to strengthen the Michael receptor's (electrophilic) group and add electron-absorbing cyanoacids to further boost the reactivity, which will enhance antioxidant and anti-inflammatory effects and lower ROS levels.

3/9/2023: Zavzpret (zavegepant)
Basic information: Pfizer developed Zavegepant, a CGRP (Calcitonin gene-related peptide) receptor antagonist, to treat adult migraineurs with and without aura acutely. CGRP is a crucial biomarker for migraine, and patients' blood levels of CGRP considerably rise during migraine attacks.
Molecular design concept: Using information from journals and patents, we compiled the structural characteristics of well-known CGRP receptor antagonists and came up with a hit that has a Ki of 0.55 nM but is also a strong CYP3A4 inhibitor and poorly soluble. 7-methylindazole was substantially more active and showed acceptable CYP3A4 inhibition, according to SAR of the benzothiophene side chain. Additionally, the addition of a fluorine atom to the quinazolinone's C-8 position produced molecule 2 (BMS- 694153). A simple SAR of the piperidine-piperidine side chain was changed to N-methylpiperidinyl-piperazine (with two protonatable nitrogen to improve water solubility) to produce BMS-742413 (i.e., the marketed drug zavegepant), despite the fact that 2 is susceptible to oxidation in aqueous solution. The article "Drug Design of the CGRP Receptor Antagonist Zavegepant" has further details about Zavegepant.

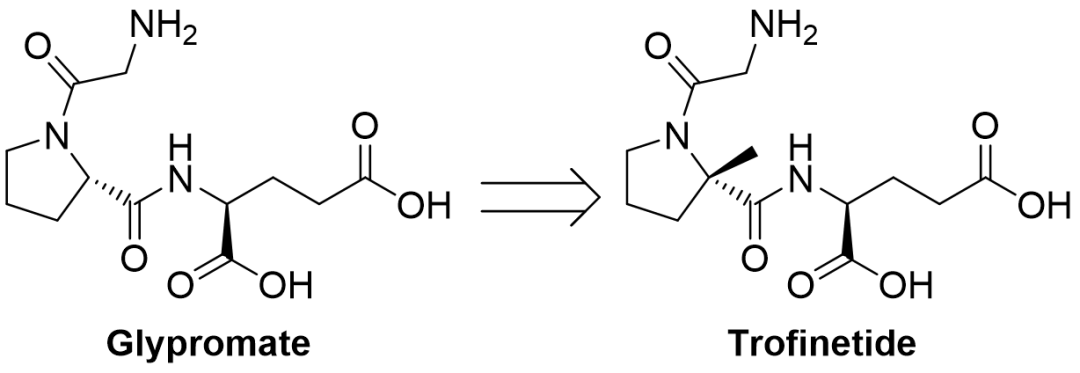
3/10/2023: Daybue (trofinetide)
Basic information: Trofinetide is the first medication (oral solution) created by Acadia Pharmaceuticals (in partnership with Neuren Pharmaceuticals, Australia) for the treatment of patients with Rett syndrome aged 2 years and older. Rett syndrome is a complex, rare neurodevelopmental disorder typically brought on by a period of normal development up until the age of 6 to 18 months, followed by significant developmental regression.
Trofinetide is a synthetic analogue of the amino-terminal tripeptide of IGF-1 (insulin-like growth factor 1) with an additional methyl substitution on proline. This improves the metabolic resistance and oral availability of trofinetide to protease activity, extending the half-life and enabling oral administration. The article "Glypromate Neuropeptide Research Review: A First-in-Class New Drug Born from a Methyl Modification" has more details on the development of the medicine trofinetide.
3/22/2023: Rezzayo (rezafungin)
Basic information: Cidara Therapeutics developed rezafungin, a weekly echinocandin (injection), to treat adult candidemia and invasive candidiasis.Rezafungin affects the integrity of the fungal cell wall by inhibiting (13)-beta-D-glucan synthase. Numerous semi-synthetic derivatives have been sold since the 1974 discovery of the first echinocandin-like (cyclic hexapeptide) antifungal medication, echinocandin B.
Molecular design concepts: Semi-synthetic echinocandins Caspofungin, Micafungin, Anidulafungin, and Rezafungin were all commercialized in the US in 2001, 2006, 2005, and 2023, respectively. Semi-synthetic antifungal medications include anidulafungin and rezafungin. By substituting an alkoxytriphenyl side chain for the naturally existing fatty acid side chain in echinocandin B, anidulafungin lessens the hemolytic impact of the drug. Rezafungin is a structural counterpart of anidulafungin in which a choline amine ether is used in place of the hemiacetalamine at the R2 position. The drug's activity in vivo is prolonged by this alteration, which also enhances the drug's stability and pharmacokinetics in solution.

3/24/2023: Joenja (leniolisib)
Informational details: An oral PI3K-kinase inhibitor called leniolisib was created by Pharming (under license from Novartis) to treat patients 12 years of age and older who have PI3K syndrome (APDS). Leniolisib inhibits PI3K- by obstructing the enzyme's active binding site. Leniolisib was more selective for PI3K-delta than PI3K-alpha, PI3K-beta, and PI3K-gamma (257-fold), as well as other kinase groups, in an enzyme activity experiment. Leniolisib decreased the activity of the pAKT pathway in cellular tests and prevented the growth and activation of B- and T-cell subsets.
Molecular design concept: Using the pan-PI3K/mTOR molecule BEZ235 as a starting point, the researchers simplified the molecule to obtain the core backbone2, then used SAR to optimize the selectivity for PI3K- to obtain compound 3, then improved the inhibition of hERG and improved its metabolic instability to obtain molecule 4, and finally used SAR once more to increase the crystal's solubility to obtain leniolisib.











