Pfizer's New Nasal Spray for Acute Migraine Treatment Approved by the FDA
The FDA approved
ZAVEGEPANT (ZAVZPRET) CAS: 1337918-83-8, the first new molecular entity approved this month, for the treatment of acute migraine on March 9. It's a brand-new CGRP inhibitor that can be used to treat migraines with and without aura. It is worth noting that this product is the first in the field of CGRP inhibitors to be made into a nasal spray for a faster onset of action.
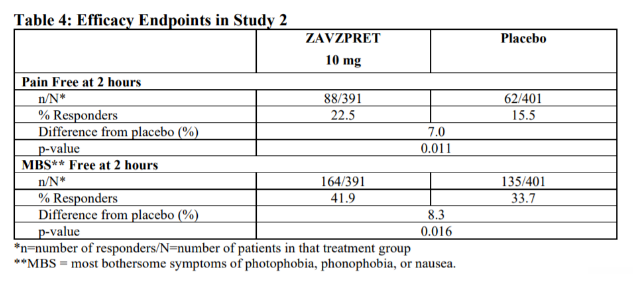
Pfizer conducted a two-line phase III trial to demonstrate the safety and efficacy of this product in order to obtain FDA approval. In Trial 1 (NCT04571060), patients with acute migraine were randomly assigned to one of two groups and treated with a single dose of this product (n=623) or placebo (n=646), with the treatment group achieving complete headache freedom (PAIN FREE) at 2 hours compared to 14.9% in the placebo group. The most bothersome migraine symptoms (photophobia, phonophobia, nausea, and so on) were eliminated in 39.6% of patients in the treatment group versus 31.1% of patients in the placebo group. The design of trial two was similar to that of trial one, and the results are as follows:
Prior to this, the FDA approved several CGRP monoclonal antibodies (Erenumab CAS 1582205-90-0, Galcanezumab CAS 1578199-75-3, Fremanezumab CAS 1655501-53-3, Eptinezumab CAS 1644539-04-7) as well as several small molecule CGRP inhibitors (Ubrogepant CAS 1374248-77-7, Rimegepant CAS 1289023-67-1, Atogepant CAS 1374248-81-3), and competition for this target was especially fierce, with many analysts previously predicting lower-than-expected sales for the heavy hitters. Most previously marketed products used immediate-release tablets or orally disintegrating tablets to get the drug to work, but CGRP inhibitors using nasal spray technology were the first of their kind. However, for migraine treatment, nasal spray delivery is a very good option; in addition to this product, approved nasal spray CGRP products for Vazegepant CAS
1337918-83-8 are expected in the future.

