Evaluate Pharma recently published an article listing the 'drugs with the best value potential' currently in development, including 10 drugs such as Merck Sharp & Dohme's ACVR2A-Fc fusion protein sotatercept, Novartis's CFB inhibitor iptacopan, and Madrigal's resmetirom, a heavy-hitting new drug in the NASH space.
Four of these substances have been gathered together: Iptacopan, Aficamten, Resmetirom, and Karxt (a zanormerin tartrate and trimethoprim combination).
1. Iptacopan
CAS No.: 1644670-37-0

Description: The first oral complement system regulatory factor B targeted inhibitor developed by Novartis, a Swiss pharmaceutical company.
Usage: Treatment of complement-driven kidney disease (CARD).
Progress: In June 2023, the CDE officially accepted the market authorization application for iptacopan and included it in the priority review process. Its indication is for adult paroxysmal nocturnal hemoglobinuria (PNH).
Patent: The compound patent WO2015009616 (A1) has entered the review process in both China and the United States.
2. Aficamten:
CAS No.: 2364554-48-1
Molecular Formula: C18H19N5O2
Formula Weight: 337.38
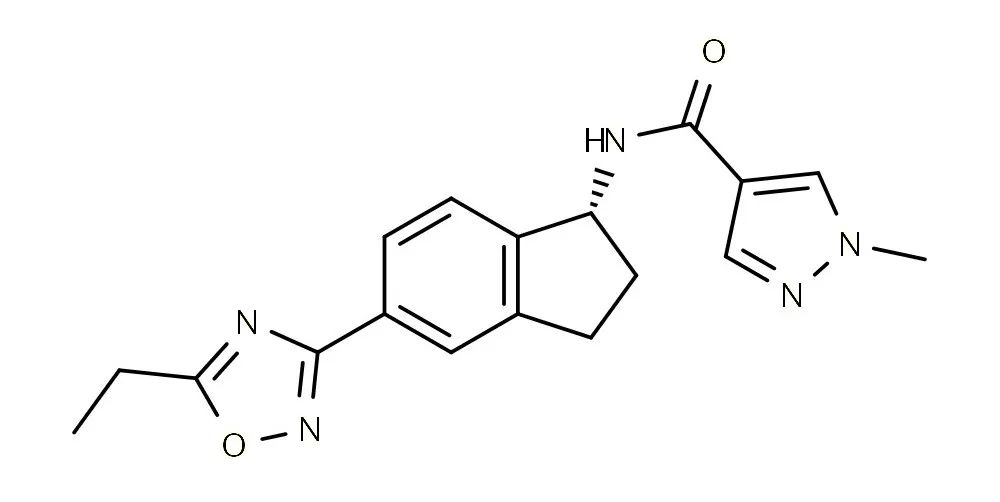
Description: A small molecule drug developed by Cytokinetics Inc.
Usage: A myosin protein complex inhibitor used to treat hypertrophic cardiomyopathy.
Patent: The compound patent WO2019144041A1 has entered the review process in both China and the United States.
3. Resmetirom:
CAS No.: 920509-32-6
Molecular Formula: C17H12Cl2N6O4
Formula Weight: 435.22
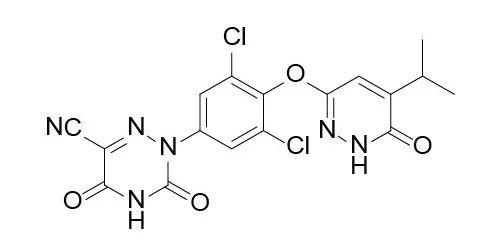
Description: A small molecule drug developed by Roche.
Usage: A THRB agonist used to treat non-alcoholic fatty liver.
Progress: Once Resmetirom is approved by the FDA, the history of the NASH treatment drug gap will be rewritten.
Patent: The compound patent WO2007009913A1 has entered the review process in both China and the United States.
4. Karxt:
Description: A compound drug developed by Karuna Therapeutics Inc.
Usage: Treatment of schizophrenia and Alzheimer's disease.
Patent: The combination patents WO2011011060A1 and WO2020069301A1 have entered the review process in both China and the United States.
Conclusion:
The article emphasizes the importance of patent retrieval and analysis in the new drug project research process. A robust patent retrieval process can avoid redundant research and patent infringement, thereby achieving patent mining and easily addressing disputes. The core patent expiration dates of the four blockbuster drugs are different, and the original research adopts different patent layouts, which will also affect the competitive landscape of the product. In the project research, the earlier you accurately retrieve and query the relevant core patents, the earlier you can carry out patent blocking, further promoting companies to build their own patent barriers.
Additionally, the article also states that its content is only an introduction to research progress in the field of medical diseases or a brief overview of research and does not make treatment or diagnostic recommendations, nor does it constitute any investment advice.


